Electric battery
- A device that stores chemical energy and converts it to electrical energy.
- a device consisting of one or more electrochemical cells with external connections for powering electrical devices
- c.f., electrochemical cells
- a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical reactions.
- Each electrochemical cell consists of two electrodes separated by an electrolyte.
- The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit.
- c.f., chemical reactions
- a process that leads to the chemical transformation of one set of chemical substances to another.
- e.g., changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms
- The flow of electrons provides an electric current that can be used to do work.
- To balance the flow of electrons, charged ions also flow through an electrolyte solution that is in contact with both electrodes.
- c.f., electrolyte solution
- a substance that is split into ions in an aqueous solution state and flows current.
- e.g., NaCl
- it does not pass current in a solid state, but can become an electrolyte because it passes current in an aqueous solution state.
- An electrolyte can be a liquid, gel or a solid substance, but it must be able to allow the movement of charged ions.
- c.f., ions
- refers to an atom or molecule that loses or gains an electron
- When the electrolyte is dissolved in water, it is divided into positive and negative ions.
- This phenomenon is called 'ionization'
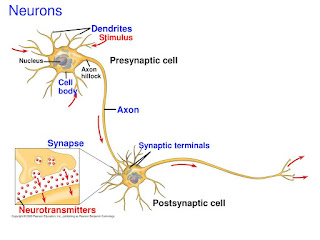
The flows of electrons (electrochemical cells)
- Electricity is a type of energy produced by the flow of electrons.
- In an electrochemical cell, electrons are produced by a chemical reaction that happens at one electrode and then they flow over to the other electrode where they are used up.
- The electrons flow from one electrode called the anode (or negative electrode) to another electrode called the cathode (the positive electrode).
- c.f., electrodes
- generally different types of metals or other chemical compounds.
- In Volta’s pile, the anode was the zinc, from which electrons flowed through the wire (when connected) to the silver, which was the battery’s cathode.
- He stacked lots of these cells together to make the total pile and crank up the voltage.
- At the anode, the electrode reacts with the electrolyte in a reaction that produces electrons.
- These electrons accumulate at the anode.
- As the chemical reaction at the anode produces electrons, to maintain a neutral charge balance on the electrode, a matching amount of positively charged ions are also produced. These don’t go down the external wire (that’s for electrons only!) but are released into the electrolyte.
- Meanwhile, at the cathode, another chemical reaction occurs simultaneously that enables that electrode to accept electrons.
- The cathode must also balance the negative charge of the electrons it receives, so the reaction that occurs here must pull in positively charged ions from the electrolyte
Increasing a battery's voltage
- To increase a battery’s voltage, we’ve got two options.
- We could choose different materials for our electrodes, ones that will give the cell a greater electrochemical potential.
- Or, we can stack several cells together.
- c.f., When the cells are combined in a particular way (in series), it has an additive effect on the battery’s voltage.
- When cells are combined in another way (in parallel) it increases the battery’s possible current, which can be thought of as the total number of electrons flowing through the cells, but not its voltage.
Battery recharging
- The flow of ions and electrons, as it takes place in some types of batteries that have appropriate electrode materials, can also go backwards, taking our battery back to its starting point and giving it a whole new lease on life
- When we connect an almost flat battery to an external electricity source, and send energy back in to the battery, it reverses the chemical reaction that occurred during discharge.
- This sends the positive ions released from the anode into the electrolyte back to the anode, and the electrons that the cathode took in also back to the anode
- The memory effect
- When you recharge some types of rechargeable batteries without sufficiently discharging them first, they ‘remember’ where they were up to in earlier discharge cycles and don’t recharge properly.
- The memory effect is strong for some types of cells, such as nickel-based batteries.
- Other types, like lithium-ion, don’t suffer from this problem.
- Another aspect
- The chemistry that makes them rechargeable also means they have a higher tendency towards self-discharge
- The lithium-ion batteries in our mobile phones
- have a pretty good self-discharge rate of around 2–3 per cent per month
- Lead-acid car batteries
- are also pretty reasonable—they tend to lose 4–6 per cent per month.
- Nickel-based batteries
- lose around 10–15 per cent of their charge per month, which is not very good
- A non-rechargeable alkaline battery only loses around 2–3 per cent of its charge per year.
Combinations of different materials of metals
- A range of materials (it used to be just metals) can be used as the electrodes in a battery
- Different materials have different electrochemical properties, and so they produce different results when you put them together in a battery cell.
- Often two or more battery cells need to be stacked to obtain the required voltage
- The lithium iron phosphate batteries (a type of lithium-ion battery) used in electric cars stack together to make high voltage systems (100 or even more volts)